 Chemistry and biology of different types of fat. Why trans-fats are so bad? This video and other related images/videos (in HD) are available for instant download licensing here:
https://www.alilamedicalmedia.com/-/g...
Chemistry and biology of different types of fat. Why trans-fats are so bad? This video and other related images/videos (in HD) are available for instant download licensing here:
https://www.alilamedicalmedia.com/-/g...
©Alila Medical Media. All rights reserved.
Voice by Ashley Fleming
Support us on Patreon and get FREE downloads and other great rewards: patreon.com/AlilaMedicalMedia/posts
All images/videos by Alila Medical Media are for information purposes ONLY and are NOT intended to replace professional medical advice, diagnosis or treatment. Always seek the advice of a qualified healthcare provider with any questions you may have regarding a medical condition.
Contrary to popular belief, not all fats cause heart diseases and are bad. In fact, most fats, in adequate amounts, are required for normal bodily functions, especially brain functions. There are also good fats that actually decrease the risks for cardiovascular diseases.
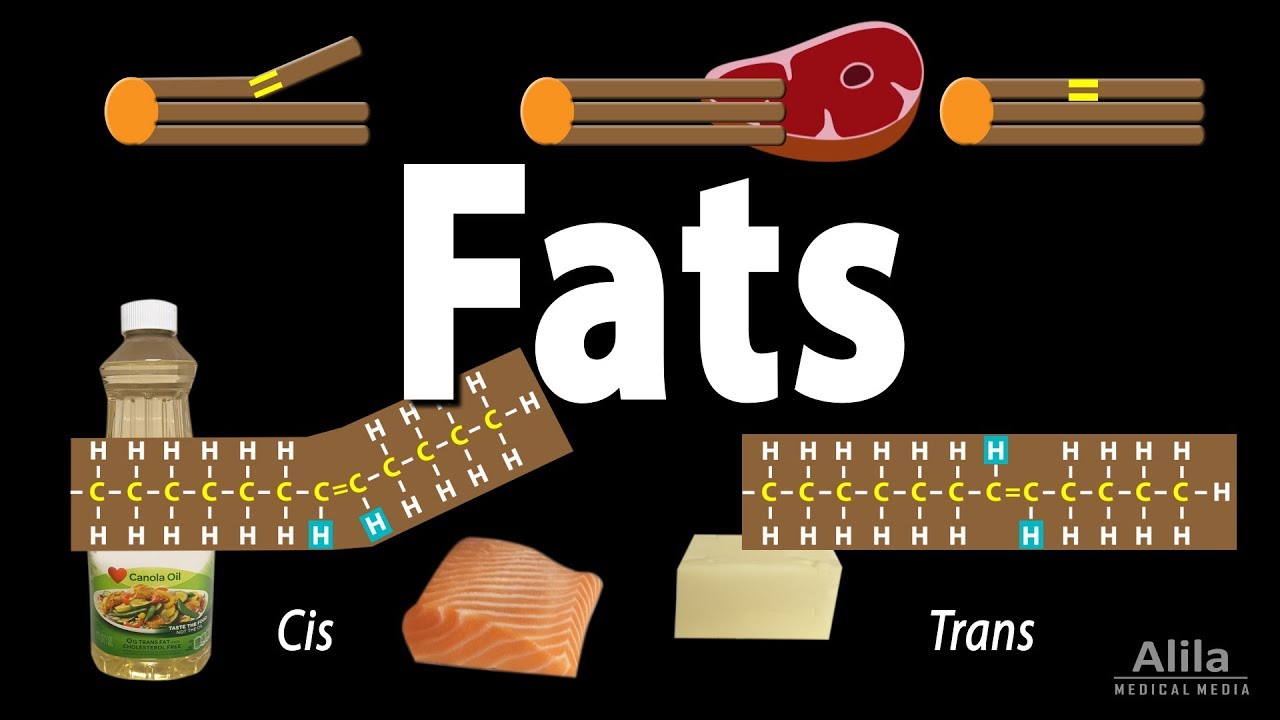
A fat molecule is composed of a glycerol head and three fatty acid tails, each of which is a long hydrocarbon chain - a carbon skeleton bound to hydrogen atoms. When all the carbons are fully bound to hydrogens, the fatty acid is said to be saturated - all the bonds between carbon atoms are single, and the hydrocarbon chain has a straight shape. A fat molecule made entirely of saturated fatty acids is a saturated fat. Due to their straight tails, saturated fats are compact and solid at room temperature.
On the other hand, when the hydrocarbon chain has fewer hydrogens, it is said to be unsaturated. Instead of binding to a maximum number of hydrogens, some carbon atoms bind to each other via a double bond. The presence of double bonds may bend the hydrocarbon chain, creating gaps between molecules, making them less compact. As a result, unsaturated fats are usually liquid at room temperature. A fat molecule that contains only one double bond is a monounsaturated fat, while one that has multiple double bonds is polyunsaturated.
Dietary fats provide fatty acids for the synthesis of the cell membrane - a vital component of all animal cells. The gaps in unsaturated fatty acids provide membrane fluidity, facilitating membrane transport and cellular signaling. While both types of fats are needed for an optimal composition of the cell membrane, too much saturated fat, which is commonly the case in a typical American diet, would make the membrane rigid and hinder cellular responsiveness. Membrane fluidity is most important in the nervous system, where neuronal response requires extremely fast cellular communication. A certain ratio of unsaturated to saturated fatty acids is also required for the formation of myelin – the insulating material that wraps around axons of neurons and speeds up the conduction of electrical signals.
The body is capable of synthesizing all the fatty acids it needs, with the exception of polyunsaturated fatty acids omega-3 and omega-6, which must be obtained from the diet. These are known as essential fatty acids.
In general, unsaturated fats are healthier than saturated fats. Unsaturated fats decrease the risks for heart disease by reducing the amount of bad cholesterol, LDL, and increasing the good cholesterol, HDL; while saturated fats increase both good and bad cholesterol. But not all unsaturated fats are equal. In fact, a type of unsaturated fat, known as trans-fat, is the unhealthiest of all!
A double bond can give rise to 2 possible configurations: cis and trans. Cis is when the 2 hydrogen atoms are on the same side of the bond, while trans is when they are on the opposite sides. A cis double bond bends the fatty acid molecule, while the somewhat more symmetric trans configuration does not. A trans-fat is therefore similar in structure to a saturated fat. More importantly, trans-fats rarely occur in nature so the body does not have the necessary enzymes to break them down. Diets rich in trans-fats increase the bad cholesterol LDL and reduce the good cholesterol HDL, having the most detrimental effect on blood vessels.
Trans-fats are found mainly in partially hydrogenated oil products, such as margarine. Because unsaturated fats are less stable and spoil faster, food manufacturers add hydrogens to make them more saturated through a process known as partial hydrogenation. This process not only prolongs shelf-life of vegetable oils, but also turns them into solid, or semi-solid products, which are preferred by commercial bakers for their low cost and wide range of different textures. Unfortunately, partial hydrogenation also converts some of the cis double bonds into trans configuration, producing trans-fats.
Unsaturated vs Saturated vs Trans Fats, Animation animated spider man | |
| 1,557 Likes | 1,557 Dislikes |
| 57,522 views views | 260K followers |
| Education | Upload TimePublished on 1 Oct 2018 |
Không có nhận xét nào:
Đăng nhận xét